ParaGard IUD Lawsuits
The crux of the lawsuit is that the device’s unique design directly contributes to fracture during removal and that there was not adequate label warning for these risks. The litigation further alleges that Teva Pharmaceuticals and CooperSurgical, who manufactured the IUD, improperly manufactured the product and failed to educate consumers about the product defect. It also alleges Teva and CooperSurgical knew the device was defective.
The Judicial Panel on Multidistrict Litigation centralized all the claims in December 2020 in the Northern District of Georgia under U.S. District Court Judge Leigh Martin May. In that holding, the panel wrote: “the actions involve sufficient common questions of fact and that centralization in the Northern District of Georgia will serve the convenience of the parties and witnesses and promote the just and efficient conduct of the litigation.”
The panel also held that centralizing the lawsuit would avoid duplicative discovery and other pretrial proceedings and the possibility of inconsistent rulings on pretrial matters.
Case Works partners with law firms working on mass tort cases, leveraging technology to ease the burden and providing the extra staff required to launch a successful mass tort campaign.
Primary Issues in the ParaGard Litigations
The core issue of the case is that the IUD has been shown to break upon removal, causing immense injury and complications from surgically repairing the injuries. The plaintiffs also allege that Teva and CooperSurgical failed to properly warn them or their doctors of the risks.
Manufacturing and Design Defects
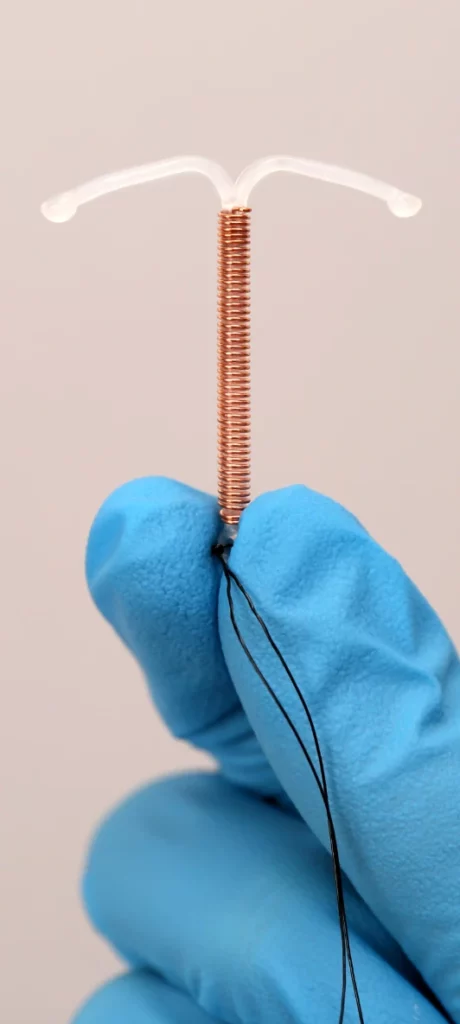
The ParaGard IUD T380 A has a unique design. It is T-shaped, which means its arms stick out straight from the center cylinder.
The plaintiffs allege that this design causes the IUD to break off, most notably during removal, even when removal takes place by a train physician or physician’s staff.
The broken pieces can then migrate to the uterus, causing organ damage and sometimes requiring surgery. In some horrific cases, women may require a hysterectomy, which leaves them unable to have children in the future.
Failure to Warn
The plaintiffs also allege that Teva’s packaging failed to include warnings about the risk of breakage. Because they failed to warn of the dangers, the manufacturers and distributors of ParaGard IUD were negligent in their duty to warn customers, the plaintiffs say.
The woman also claimed that the companies have violated their informed consent rights as they were unaware of the risk. The suit also alleges that rather than remedying the defects or warning patients, Teva and CooperSurgical continued to advertise only the benefits of the product.
Who are the Defendants in the ParaGard IUD Litigation?
The primary plaintiffs are Teva Pharmaceuticals and CooperSurgical Inc., who manufactured ParaGard.
Teva Pharmaceutical Industries Ltd.
Teva Pharmaceuticals is an Israel-based global pharmaceutical company with about 35,000 global employees, according to a Yahoo profile.
It has been in business since 1901 and has a product portfolio of more than 3,500 products. It is the world’s largest manufacturer of generic products and also specializes in late-stage development programs for disorders of the central nervous system.
It made the ParaGard IUD until it sold the product to CooperSurgical Inc. in 2017. Its net revenues in 2021 were about $16 billion, according to its financial statements.
CooperSurgical Inc.
CooperSurgical is a California-based global medical equipment and device company specializing in women’s products, according to a Bloomberg profile.
The company has 1,700 employees and manufactures more than 600 products. CooperSurgical is one business unit of The Cooper Companies, which was incorporated in 1990 and trades on the NYSE under the symbol COO.
The other business unit of Cooper Companies is Cooper Vision. According to its financial statements, Cooper Companies’ 2021 revenues were about $3 billion.
Has ParaGard been recalled?
No, the FDA has not recalled ParaGard. However, it sent a warning letter to CooperSurgical on Feb. 12, 2021.
The letter states that the Office of Prescription Drug Promotion of the FDA had watched a ParaGard promotional video that misrepresented the brand.
The letter says, “The video is false or misleading in that it presents efficacy claims for ParaGard, but fails to communicate any risk information associated with its use. Thus, the video misbrands ParaGard within the meaning of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and makes its distribution violative. 21 U.S.C. 352(a), (n); 321(n); 331(a). See 21 CFR 202.1(e)(5).”
The letter also says that the company failed to submit the video at the time of initial dissemination as “required by 21 CFR 314.81(b)(3)(i). These violations are concerning from a public health perspective because this promotional communication creates a misleading impression about the safety of ParaGard.”
Later in the letter, the OPDP says, “This misleading presentation is particularly concerning from a public health perspective due to the serious and potentially life-threatening risks associated with the drug, such as those contained in the WARNINGS AND PRECAUTIONS section of ParaGard’s PI.”
The OPDP further notes that it had previously expressed concerns about ParaGard’s promotional materials in a letter issued on June 25, 2019. The OPDP issued that letter in response to a consumer-based television advertisement that also touted the benefits of ParaGard while omitting a discussion of the risks.
It requests that CooperSurgical submit a plan to discontinue the use of that video and any others that make similar claims without including truthful information on its risks.
It also requests that the company disseminate “corrective communications” to the audience. It said that failure to do so might result in regulatory action.
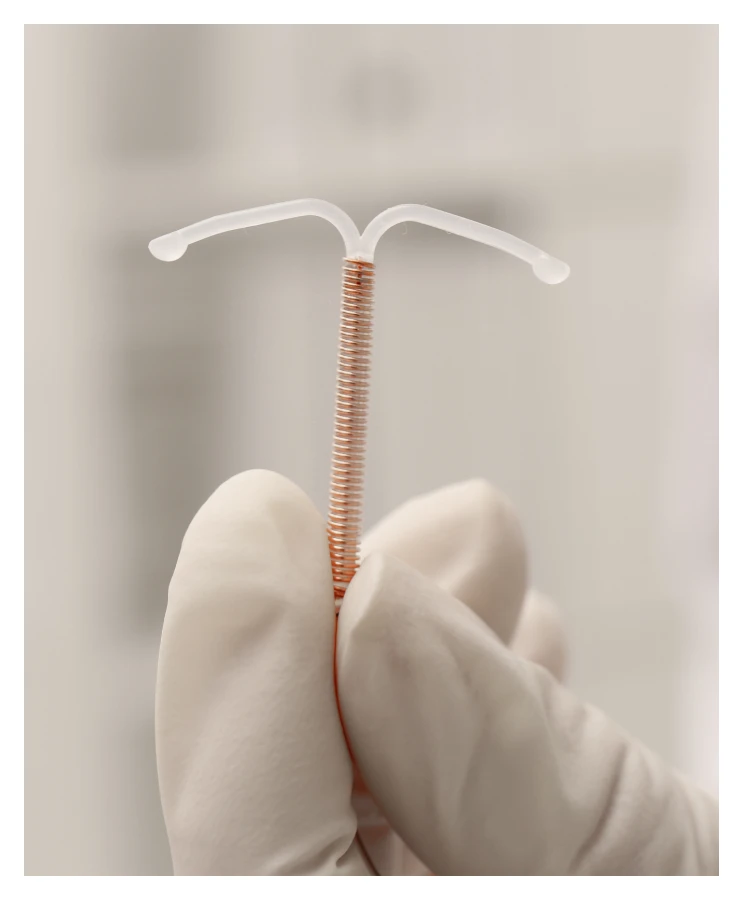
What is the ParaGard IUD
ParaGard IUD is a birth control device that was designed to be effective for up to 12 years.
It is a T-shaped plastic frame that a doctor inserts through the cervical opening. It works through its copper wire that is coiled around the device and prevents the sperm from fertilizing the egg.
It is the only copper IUD available in the United States. Teva Pharmaceutical originally manufactured the device, which the FDA approved for use in 1984.
Complications
The ParaGard IUD can result in several complications, according to the FDA.
The arms of the device can break off and embed in the uterus.
If this happens, the embedding can damage organs. The break-off occurs most often during the removal process and can cause pain, infection, and infertility. It also usually requires a second surgical removal.
One of the first women to file a ParaGard lawsuit was Vanesha Johnson, who filed in New York in early 2020, according to Medtruth. She alleged that ParaGard broke off when her doctor removed it. Her lawsuit says that she had to undergo surgical removal and lost her reproductive health, pain, suffering, mental anguish, loss of enjoyment of life, and significant medical expenses.
Uterine perforation occurs in 1 to 2 out of 1,000 insertions of a copper IUD,
according to an article in the International Journal of Women’s Health.
These perforations often go undetected until they cause damage. When these perforations occur, the copper IUD can erode into the bladder and intestinal tract, and also possibly damage adjacent organs. the Journal says.
Pelvic infections are another potential complication.
They bring a serious risk of tubal damage, which can lead to infertility and hysterectomy.
The infections also can lead to sepsis and death. In some cases, the woman may never realize she has an infection, yet her uterus and other reproductive organs may still be damaged, according to Women’s Health Specialists.
Although ParaGard is about 99 percent effective, pregnancies do occur sometimes.
An intrauterine pregnancy is a normal pregnancy. The doctor will generally try to remove ParaGard when a woman becomes pregnant.
If ParaGard is in the uterus, it can cause an increased risk of miscarriage, septic shock, and sepsis. However, removing ParaGard also sets up the risk of breakage, especially if the strings have curled up into the cervix.
According to the FDA, women who use the ParaGard IUD and become pregnant also are more likely to have ectopic pregnancies than women in the general population who become pregnant.
Ectopic pregnancies occur “when a fertilized egg implants and grows outside the main cavity of the uterus,” according to the Mayo Clinic. Most frequently, an ectopic pregnancy occurs in the fallopian tube, although an ectopic pregnancy can occur in other areas of the body. Ectopic pregnancy is the leading cause of maternal death in the early stages of pregnancy.
One woman who is suing the ParaGard manufacturers became pregnant while using the IUD. She made an appointment with her doctor to have ParaGard removed per the manufacturer’s instructions. However, before the appointment, she had pain and hemorrhaging, only to discover an ectopic pregnancy. Her baby did not make it, and the doctor had to remove one of her fallopian tubes. She also said that had she lived further from the hospital than five minutes, she could have died of sepsis, according to a recent CBS 12 news story. The loss of the fallopian tube makes her chances of conceiving again very low, the report said.
The body produces more white blood cells as a result of having the IUD in place.
“The body’s normal reaction to a low-grade inflammation is to produce scar tissue to protect the area from infection,” according to Women’s Health.
This scar tissue can damage a woman’s reproductive health, Women’s Health says.
The FDA says that theoretically, ParaGard can make Wilson’s Disease worse.
Wilson’s Disease is a rare inherited disease that causes copper to accumulate in the brain, liver, and other vital organs.
It typically strikes people between 15 and 35.
Help in Preparing Claims
Case Works provides expert case development services for mass tort and personal injury cases. If your firm needs help preparing ParaGard claims for settlement or trial, reach out to us today.
Administrative tasks related to litigation can be time-consuming and take staff time and energy away from focusing on other clients.
We’ve developed a streamlined system that enables us to handle all your mass tort case development needs so that your attorneys and staff members can focus on other important areas in litigation.
We can customize our services to suit your needs. We provide expert services in:
Save Time &
Reduce Costs
Client management
and communication
Medical records retrieval and management
Case operations management
Medical record
review
Plaintiff form
creation
Client diagnostic service
Case filing
As a seamless extension of your firm, our team can help your firm prove claims and get them ready to settle. Contact us today to find out how we can complete the case review for ease of settlement!